From high-tech laboratories to the final rinse in a car wash, the uses of deionised water are incredibly varied. In any situation where even the tiniest impurity matters, this highly purified water is the secret ingredient. It’s essentially a blank canvas, stripped of the mineral ions that are ever-present in tap water, making it perfect for jobs where mineral residue could ruin the finish or disrupt a delicate process.
So, What Makes Deionised Water Different?
Think of your regular tap water as a cup of tea. It might look clear, but it's full of dissolved 'flavours' – in this case, minerals like calcium, magnesium, and sodium. These are what we call ions. While perfectly fine for us to drink, they can cause real headaches in other settings. They’re the culprits behind limescale in your kettle, they can interfere with precise chemical reactions, and they even allow water to conduct electricity.
Deionisation is the process that removes all of that 'flavour'. It filters out those mineral ions, leaving behind nothing but pure, unadulterated H₂O.

The end result is deionised (DI) water—a remarkably pure solvent. By getting rid of those charged particles, the water stops conducting electricity and, crucially, leaves absolutely no mineral spots behind when it evaporates. This turns it into the ultimate "blank slate" for tasks demanding absolute cleanliness and purity.
To get a clearer picture, let's compare the two side-by-side.
Tap Water vs Deionised Water At a Glance
This table breaks down the fundamental differences between the water from your tap and its ultra-pure counterpart.
| Characteristic | Tap Water | Deionised Water |
|---|---|---|
| Mineral Content | Contains dissolved minerals (calcium, magnesium, etc.) | Virtually all mineral ions removed. |
| Purity Level | Variable, contains impurities and additives like chlorine. | Exceptionally high purity (99.9% pure H₂O). |
| Electrical Conductivity | Conductive due to mineral ions. | Non-conductive (or very low conductivity). |
| Residue on Evaporation | Leaves mineral deposits (limescale, spots). | Evaporates cleanly, leaving no residue. |
| Common Uses | Drinking, cooking, cleaning. | Laboratory work, manufacturing, automotive detailing. |
As you can see, while tap water is great for everyday life, deionised water is a specialist tool designed for jobs where purity is non-negotiable.
The Science Behind the Purity
At its heart, making deionised water involves a clever process called ion exchange. Untreated water is passed through special resin beads that are engineered to act like magnets for mineral ions.
These resins cleverly swap the unwanted ions (like calcium and sodium) for harmless hydrogen (H+) and hydroxyl (OH-) ions. These then immediately combine to form pure water (H₂O).
By removing virtually all dissolved mineral salts, the deionisation process creates water that is non-conductive and exceptionally pure. This characteristic is fundamental to its role in preventing contamination and ensuring operational efficiency across numerous sectors.
It’s a highly effective system and the cornerstone of producing high-purity water for critical applications. To see exactly how this technology works, you can find a more detailed explanation of how the ion-exchange system works.
Across the UK's industrial and scientific landscape, deionised water is indispensable. In laboratories, its purity is essential for ensuring experiments are accurate and repeatable. Chemical manufacturers and biotech firms depend on it to mix formulas without fear of contamination. For many, it's a more cost-effective choice than distilled water, as the deionisation process is far less energy-hungry than distillation. This foundational purity is what makes the different uses of deionised water so critical.
Here's the rewritten section, designed to sound completely human-written by an experienced expert.
Why Science and Labs Depend on Pure Water
Walk into any scientific laboratory, whether it’s a university research centre or a top UK pharmaceutical firm, and you’ll find that precision is the name of the game. Even the tiniest contaminant can throw off an experiment, wasting valuable time, money, and leading to conclusions that are simply wrong. In this world of high stakes, deionised water is the unsung hero—it provides the perfect "blank canvas" for discovery.
Think about what would happen if you tried to mix a sensitive chemical solution with ordinary tap water. You’re not just adding H₂O; you're also introducing a whole cocktail of stray mineral ions like calcium, magnesium, and sodium. These uninvited guests can kick off unwanted side reactions, changing the very chemistry you’re trying to study and making your results useless. Deionised water completely removes that variable, guaranteeing that the only things reacting are the ones you put there on purpose.
Getting Accurate and Repeatable Results
The reliability of a lot of high-tech lab equipment hangs on the quality of the water it uses. Take High-Performance Liquid Chromatography (HPLC), a powerful technique for separating and identifying components in a mixture. Even minuscule ionic contaminants in the water can mess with the detector readings, creating false "ghost peaks" on the results chart or shifting timings, which can lead to a completely flawed analysis.
It’s a similar story in molecular biology. Techniques like the Polymerase Chain Reaction (PCR), which is used to amplify tiny bits of DNA, are incredibly sensitive. If foreign ions—especially heavy metals—are present, they can shut down the enzymes that make the reaction work, causing the entire experiment to fail.
In a lab, consistency is everything. Deionised water ensures every experiment begins from the exact same baseline. That’s what makes results trustworthy, repeatable, and scientifically sound. Without it, researchers are left fighting an invisible enemy.
The Importance of a Spotless Clean
Beyond its use in the experiments themselves, deionised water is absolutely critical for keeping the lab environment pristine. When it comes to cleaning delicate glassware like beakers, flasks, and petri dishes, using tap water is a rookie error. As it dries, it leaves behind a chalky film of mineral deposits—what we call spotting or scaling.
This residue can easily contaminate the next experiment. Even worse, if the glassware is sterilised with these deposits still on it, they can get baked onto the surface, making them a nightmare to remove. Because deionised water has no dissolved minerals, it evaporates without a trace, leaving a perfectly spotless surface. This means the glassware isn't just visually clean; it's chemically pure and ready for the next sensitive procedure.
The crucial uses of deionised water in science really boil down to a few key areas:
- Preparing Solutions: It’s the ideal pure, non-reactive solvent for creating all sorts of chemical reagents, buffers, and standards.
- Running Equipment: It's essential for running autoclaves, cell culture incubators, and other analytical instruments without causing limescale build-up or ionic interference.
- Final Rinsing: It delivers a residue-free final rinse for all lab equipment, preventing any cross-contamination between experiments.
For many of the UK's leading biotech and research facilities, the path to a major breakthrough often starts with something surprisingly simple: the purest water they can get.
Deionised Water in UK Manufacturing and Industry
Step away from the lab, and you'll find that deionised water is one of the unsung heroes of UK manufacturing. On the factory floor, everything comes down to consistency, quality, and keeping expensive machinery running. This is where ultra-pure water becomes a critical, if invisible, part of the process, protecting everything from microscopic threats lurking in tap water.

Whether it’s the delicate art of making microchips or the precise science of formulating premium cosmetics, deionised water is quietly working in the background to improve efficiency and guarantee product quality. Its influence stretches across a huge range of sectors that are the backbone of the UK economy.
Protecting Precision Electronics
In the world of electronics manufacturing, a single speck of dust can spell disaster. A stray mineral ion can be just as bad. During production, circuit boards and microchips need a thorough wash to get rid of flux residues and other contaminants. Using regular tap water for this is a non-starter.
Why? Because tap water leaves behind a film of dissolved salts as it evaporates. These mineral deposits are conductive, creating tiny, unwanted bridges for electricity to cross. The result is short circuits and dead devices. Deionised water, on the other hand, is an excellent insulator. It rinses sensitive components perfectly clean without leaving a trace of conductive residue, ensuring every chip and board works exactly as it should.
Ensuring Stability in Cosmetics and Pharmaceuticals
Ever noticed ‘Aqua’ at the top of an ingredients list for a face cream? You can bet that’s highly purified water. In cosmetics, pharmaceuticals, and countless other formulations, deionised water is the go-to solvent because it's a completely neutral, stable base.
With no mineral ions to interfere, deionised water prevents unexpected chemical reactions with other ingredients. This ensures the final product is stable, safe, and has a dependable shelf life.
This level of purity is absolutely essential in pharmaceutical production, where even the tiniest impurity could change how a drug works or compromise its safety. To see how other high-stakes sectors rely on this, have a look at our guide to the seven important industries that use ultrapure water.
Advanced Cooling and Equipment Protection
Think about the immense heat generated by heavy industrial equipment or high-powered lasers. They all need robust cooling systems to keep running safely, and using tap water here is asking for trouble.
The minerals in tap water quickly build up as limescale, clogging pipes and choking the system. Cooling efficiency plummets, and you run the risk of catastrophic overheating. Deionised water is completely free of these minerals, so limescale simply can't form. Better yet, its non-conductive nature adds a vital layer of safety—if a leak occurs, it won’t cause an electrical short. This simple switch protects incredibly expensive machinery and slashes costly downtime.
The scale of this is huge. In the UK, non-household water consumption—which covers countless industrial processes like these—hits a staggering 2,757 megalitres per day. Industries that depend on pure water make up a significant chunk of that figure, proving just how woven into our national industrial fabric it truly is.
Getting More from Your Motor: A Boost for Car Care
You might think deionised water is just for science labs, but its real-world benefits are just as powerful in your own garage. For car lovers and professionals alike, making the switch from tap water is one of the smartest moves you can make. It's a simple change that protects your vehicle's most critical parts, saving you from future headaches and expensive repairs.
Let's start with your car's battery. A standard lead-acid battery often needs topping up, and reaching for the tap is a common mistake. Tap water is full of minerals like calcium and magnesium, which might seem harmless, but inside a battery, they cause havoc.
These minerals cling to the lead plates, forming a crust that slowly strangles the battery's ability to hold a charge. This build-up, called sulphation, is a primary reason why batteries lose power and eventually fail.
Using deionised water to top up your battery is a non-negotiable for extending its life. With no minerals to get in the way, the internal chemistry works just as it should, ensuring reliable starts and maximum performance.
Keep Your Engine Cool Under Pressure
Your car's cooling system is another place where tap water is a real problem. Those same minerals—calcium and magnesium—are the culprits behind limescale. Over time, they build up inside the radiator, water pump, and the narrow channels of your engine block.
Think of it like plaque in an artery. This scale restricts the flow of coolant and acts as an insulator, trapping heat instead of releasing it. It’s a fast track to engine overheating, which can lead to warped cylinder heads and catastrophic failure.
Using deionised water in your coolant mix completely sidesteps this issue. Your cooling system stays clean and clear, allowing it to do its job properly and keep your engine running at the temperature it was designed for.
The Secret to a Perfect, Spot-Free Shine
Ever wonder how professional detailers get that flawless, mirror-like finish on a car? Their secret weapon is often a final rinse with deionised water. It’s the key to a truly showroom-worthy look.
When you rinse a car with tap water, every droplet that evaporates leaves behind a tiny mineral deposit. These are the annoying white water spots that ruin the look of a freshly washed vehicle.
Because deionised water is just pure H₂O, it evaporates without a trace. No minerals, no residue, no spots. This means you don't have to frantically dry the car with a towel, which can introduce fine scratches into the paintwork. For anyone serious about a perfect finish, understanding the role of pure water in car detailing is an absolute game-changer.
Deionised vs Distilled Water: What's the Difference?
It’s a common mix-up, but deionised and distilled water are not the same thing. While they’re both highly purified, they’re produced in completely different ways, which gives them distinct properties. Getting this right is crucial for a lot of jobs, so let's break it down.
As we've covered, making deionised water is all about chemical filtration. The water flows through special ion-exchange resins which act like super-magnets for minerals. They grab hold of charged ions like calcium, magnesium, and sodium, and leave you with pure H₂O.
Distillation, on the other hand, is a physical process. It’s essentially the water cycle in a box. You boil water to create steam, then capture that steam and cool it back down into liquid. This process leaves nearly everything else behind – minerals, organic compounds, and even bacteria can’t make the journey as steam.
Purity vs Sterility: The Key Distinction
So, what’s the real difference? It all comes down to what gets removed. Deionisation is brilliant at stripping out mineral salts, but it won’t touch uncharged organic molecules like bacteria or viruses. Distillation, however, gets rid of both, resulting in water that’s not just pure but also sterile.
This is why their uses are so different. For medical tasks or sensitive lab experiments where you absolutely cannot have any microbial contamination, distilled water is the only way to go. Using deionised water in a sterile environment could ruin your results or, worse, cause harm. If you're interested in learning more about these high purity levels, have a look at our guide on why ultrapure water is not your drinking water.
The image below gives a great visual of just how effective deionisation is at tackling mineral-related problems.
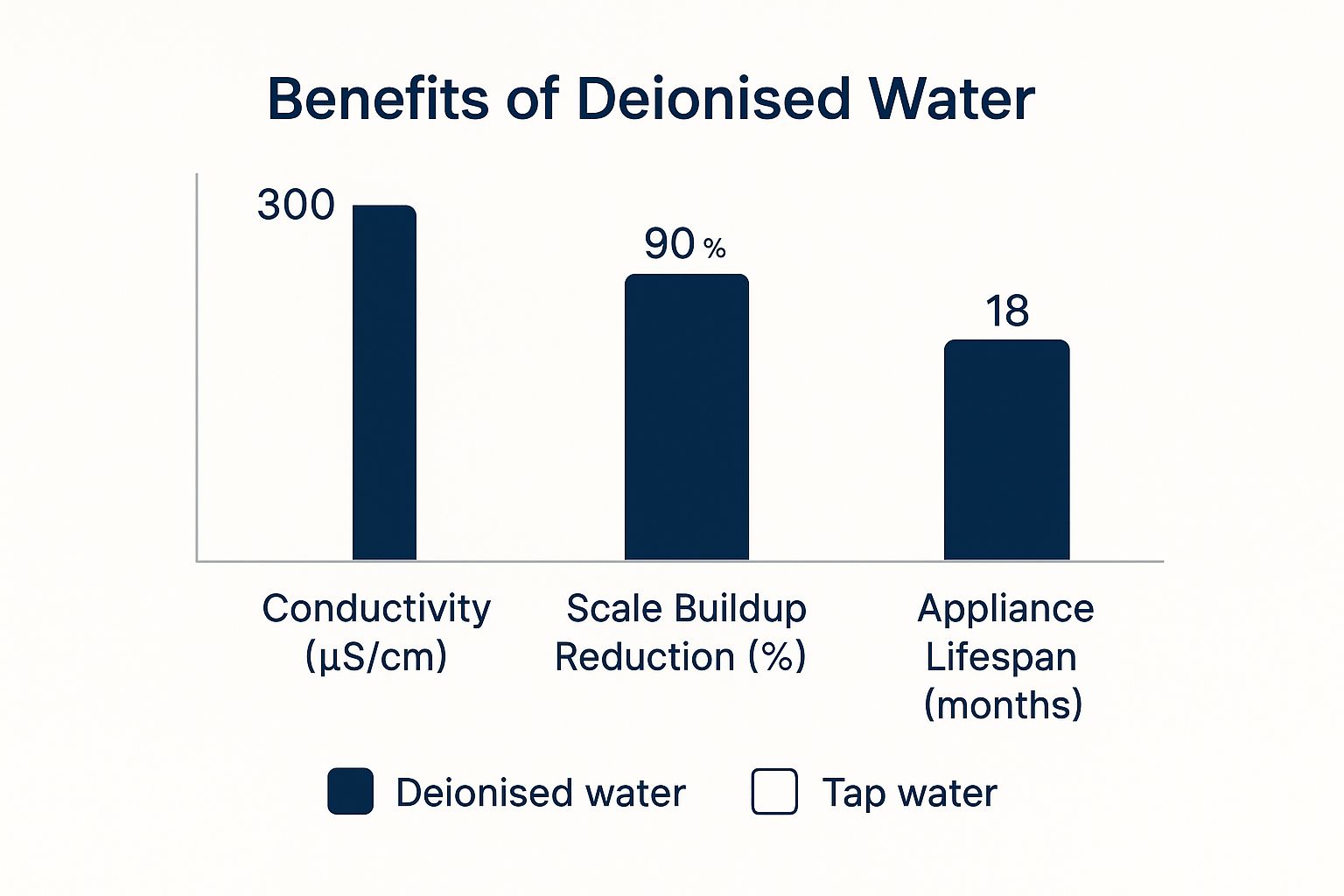
As you can see, that massive drop in conductivity and scale shows exactly why removing those mineral ions is so important for keeping appliances and equipment running smoothly.
A Practical Comparison
For the vast majority of industrial, automotive, and cleaning tasks—which make up the most common uses for deionised water—sterility simply doesn’t matter. The main goal is to stop limescale build-up and avoid mineral spots. For this, the more affordable and energy-efficient process of deionisation is the perfect fit.
Choosing the Right Water Type for Your Needs
To make the decision clearer, it helps to see the different water types side-by-side. Each one has its place, and picking the right one depends entirely on what you're trying to achieve.
| Feature | Tap Water | Deionised Water | Distilled Water |
|---|---|---|---|
| Purity Level | Contains minerals, chlorine, and trace contaminants. | Minerals are removed, but organic matter may remain. | Extremely high purity; free of minerals and microbes. |
| Production Method | Municipal treatment (filtration and disinfection). | Chemical filtration using ion-exchange resins. | Physical separation via boiling and condensation. |
| Best For | Drinking, cooking, and general household cleaning. | Preventing limescale in batteries, coolers, and for spot-free rinsing. | Sterile medical uses, lab experiments, and CPAP machines. |
| Primary Benefit | Safe to drink and readily available. | Prevents mineral deposits and corrosion. | Guarantees sterility and avoids contamination. |
| Cost | Lowest cost. | More affordable than distilled water. | Higher cost due to energy-intensive production. |
Ultimately, the choice is straightforward. If you need to stop mineral build-up, deionised water is your best bet. If you need water that is completely sterile and free from any living organisms for medical or sensitive scientific work, you must use distilled water.
Practical Tips for Handling and Storage
Getting your hands on deionised water is just the first step. To keep it in its ultra-pure state, you need to be smart about how you handle and store it. Think of DI water as a sort of purity sponge; because it’s stripped of all its ions, it’s incredibly eager to pull them back in from anything it touches.
This 'ion-hungry' nature is what makes it so useful, but it also presents a challenge. It's so aggressive in its quest for ions that it will literally leach them from certain materials. Store it in a copper or iron container, for example, and you’ll have a problem. The water will pull metal ions straight from the container walls, ruining its own purity and slowly degrading the container itself.

The solution is simple: always use containers made from materials that won't react with the water.
Choosing the Right Storage Container
To keep your deionised water in pristine condition, the container you choose is everything. Here’s what you need to know:
- Go for Plastic: The gold standard is high-density polyethylene (HDPE), but other chemically resistant plastics work well too. These materials are inert, meaning they won't give up any contaminants and compromise your water.
- Seal It Tight: Deionised water will even absorb impurities from the air. Carbon dioxide is the main culprit. As soon as it dissolves into the water, it forms a weak carbonic acid, which messes with the water's purity and pH. A tightly sealed lid is your best defence.
- Keep It in the Dark: Store your containers away from direct sunlight. UV rays can encourage the growth of algae or bacteria, which is the last thing you want when you need pure water for sensitive uses of deionised water.
Purity Checks and a Word on Drinkability
It’s a question that comes up a lot: can you drink deionised water? The short answer is that while it isn't toxic, you really shouldn't. The deionisation process strips out everything, including essential minerals like calcium and magnesium that our bodies actually need.
Deionised water's lack of minerals gives it a distinctly flat, unappealing taste. Its true value is as a high-purity solvent for technical and industrial jobs, not as a source of hydration.
If you’re working on a project where purity is paramount, you’ll want to be sure your water is still up to spec. The easiest way is with a handheld Total Dissolved Solids (TDS) meter. A fresh, properly stored batch of DI water should give you a reading close to zero, letting you know it’s pure and ready to go.
Got Questions? We’ve Got Answers
To finish up, let's tackle a few of the questions we hear all the time about deionised water. Getting these common queries cleared up should give you the confidence to put DI water to work, whatever your project.
So, Can I Drink It?
This is easily the most common question, and the short answer is no, you really shouldn't. While a small sip won’t harm you, making a habit of drinking deionised water is a bad idea.
The deionisation process is so effective that it strips out everything, including beneficial minerals like calcium and magnesium that our bodies need to function properly. Because the water is so pure, it's 'hungry' for minerals and can potentially pull them from your body. Stick to tap or bottled water for hydration and leave the DI water for your equipment.
Is It Okay for My Steam Iron or Humidifier?
Yes, absolutely! In fact, it’s the best thing you can use. Using deionised water in appliances like steam irons, humidifiers, or CPAP machines is a brilliant move. It completely stops the build-up of limescale—that crusty white stuff—that inevitably clogs and ruins them.
Think of it this way: by preventing limescale, you're not just helping your appliances run better, you're massively extending their lifespan. It's a simple, cheap way to protect your kit.
How Can I Tell if My Deionised Water Is Still Good?
It's true that the purity of deionised water can dip over time, especially if the container is left open. It loves to pull in carbon dioxide from the air, which can slightly change its chemistry. For casual uses like filling your iron, this tiny change won't matter a bit.
But if you're using it for something more sensitive, the best way to be sure is with a Total Dissolved Solids (TDS) meter. These handy gadgets give you an instant purity reading. To keep your water in top condition, always store it in a clean, tightly sealed plastic container and keep it out of direct sunlight.
For professionals needing a consistent supply of ultra-pure deionised water on the go, 24 Pure Water provides a UK-wide network of 24/7 self-service filling stations. Find out more about getting high-quality water for your business at https://24purewater.co.uk.
